Hello Glasgow! CCS goes to ISPOR EU 2025
It’s that time of the year again — ISPOR EU is taking place from November 9th to 12th, 2025 in Glasgow Scotland. CCS will be there presenting an exciting array of work spanning transportability analysis, target trial emulation, simulation-guided trial design and meta-analysis.
You can download the posters we’ll be presenting by clicking on the poster images below.
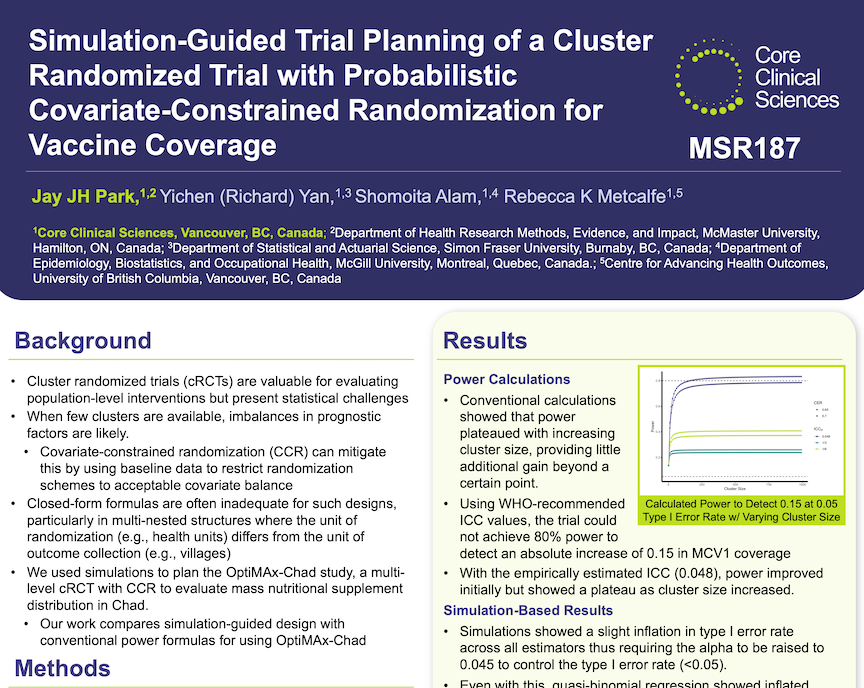
Improving design of cluster-randomized trials using statistical simulations: A case study in rural Chad
Simulation-guided design of individually-randomized clinical trials is frequently used to optimize trial operating characteristics and efficiency. However, its application to cluster-randomized trials has been limited. Instead, design of these trials often relies on closed-form sample-size calculations which may not be able to fully reflect the trials’ complexity. In this study, we compared statistical simulations and convention sample-size calculations for planning of a cluster-randomized trial with a complex data structure in rural Chad.
Read the preprint on Arxiv.
Alternatives to randomization: Using the target trial emulation framework to plan clustered, prospective studies when randomization isn’t possible
Target trial emulation (TTE) offers a powerful framework for analyzing real-world data, but its use in studies designed to mirror cluster randomized trials remains rare. In this project, we applied simulation-based methods to plan a non-randomized study in Niger aimed at evaluating whether adding nutritional supplements to an existing immunization program could improve pentavalent vaccine coverage. The results informed our choice of beta-regression as the primary analysis method, ensuring reliable inference despite the absence of true randomization. This work demonstrates how simulations can sharpen study design decisions in TTE, especially when evaluating community-level interventions.
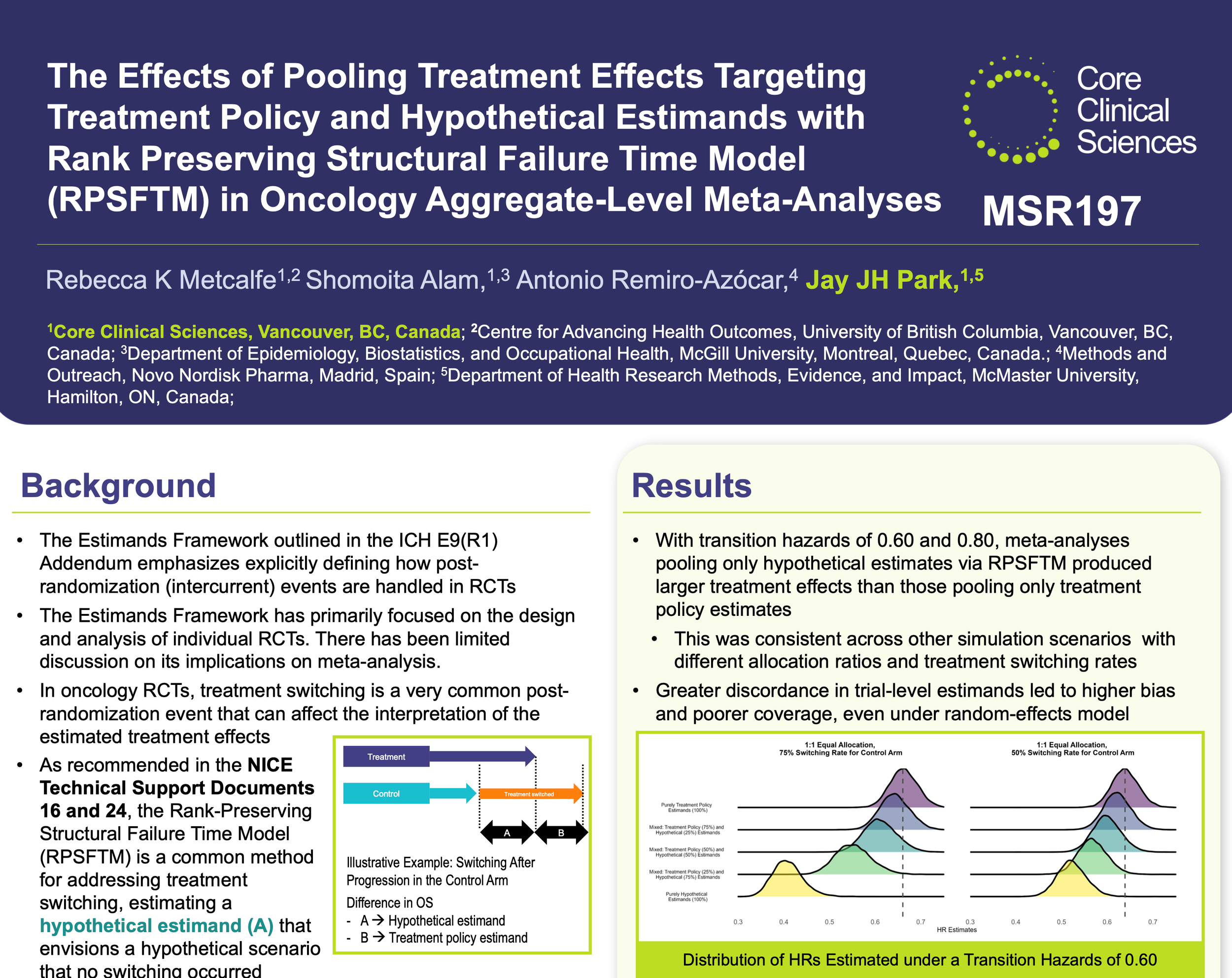
Evidence synthesis in the estimands era: What are the implications of combining different estimands in a meta-analysis?
The ICH E9(R1) addendum introduced the estimands framework to clarify how intercurrent events—like treatment switching—should be handled in clinical trials. Yet, its application to meta-analysis has received far less attention. In this study, we used simulations to examine how pooling together trial results that target different estimands can bias meta-analytic findings in oncology, where treatment switching is common. We simulated randomized clinical trials allowing control-arm patients to switch to the intervention and analyzed each using estimators targeting either a treatment policy or hypothetical estimand. Combining estimates from different estimands led to pooled results that failed to represent either estimated of interest.
The full paper was published in Research Synthesis Methods last month.
Improving feasibility and accuracy of transportability analysis: New methods with time-varying censoring weights and aggregate-level data in the target population.
Transportability analysis helps determine whether findings from a study can be applied to a different population by adjusting for differences in key effect modifiers. However, most current methods assume access to individual-level data for both the study and target populations—an assumption that limits feasibility. In this work, we introduce Target Aggregate Data Adjustment (TADA), designed to transport treatment effects in survival settings while addressing both censoring bias and population differences using only target aggregate-level data. Using simulations and a lung cancer trial application, we show that TADA can reduce bias under realistic censoring conditions.